Southeastern Retina Associates is one of 100 sites across the country partnering with the National Institutes of Health (NIH) in a study of the use of the drug Eylea as an injectable eye treatment for diabetic patients with vision problems.
The study will evaluate the effectiveness of the new treatment compared to laser therapy, the current gold standard treatment for a complication of diabetic retinopathy in which patients suffer bleeding in the retina. The condition can result in blindness if not treated successfully.
Patients taking part in the study will get either injections of Eylea, laser therapy, or simply be observed for changes.
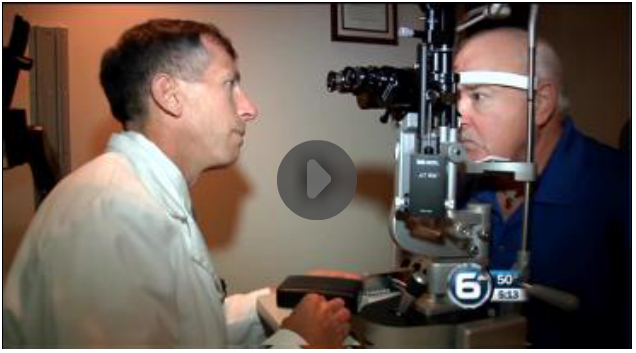
Steve Perkins, M.D., of Southeastern Retina Associates is recruiting diabetics whose vision remains relatively undamaged to participate in the study. Injections of Eylea are free, though patients or their insurance may be required to pay other costs involving regular eye care.
Patients interested in participating in the study may call 865-588-0811 for more information.